Antioxidant Deactivation on Graphenic Nanocarbon Surfaces
Xinyuan Liu,1 Sujat Sen,1 Jingyu Liu,1 Indrek Kulaots,2 David Geohegan,3 Agnes Kane,4 Alex A. Puretzky,3 Christopher M. Rouleau,3 Karren L. More,5 G. Tayhas R. Palmore,2 and Robert H. Hurt2
1-Dept Chemistry, Brown University
2-School of Engineering, Brown University
3-Center for Nanophase Materials Sciences, Oak Ridge National Laboratory
4-Dept Pathology & Laboratory Medicine, Brown University
5-Shared Research Equipment Facility, Oak Ridge National LaboratoryPDF of article published in Small 7, 2775–2785 (2011).
See this and other highlights at the (CNMS News and Highlights Page).
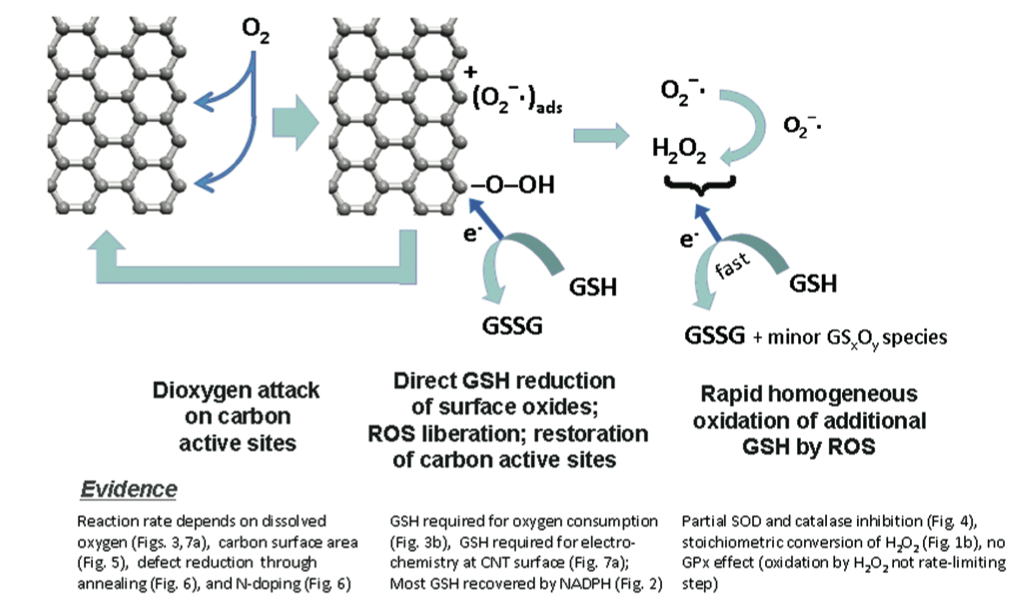
Proposed reaction mechanism by which carbon nanomaterial edges react with oxygen molecules to produce reactive surface-bound oxygen intermediates (shown) and free reactive oxygen species (such as O2- and H2O2) which deplete key physiological antioxidants (here glutathione, GSH) by producing oxidized byproducts (here, the oxidized dimer GSSG).
Achievement
This article reports a direct chemical pathway for antioxidant deactivation on the surfaces of carbon nanomaterials, comparing the relative biological reactivity of different materials in the graphenic carbon family. Glutathione, a key physiological antioxidant, was shown to be depleted by all the graphenic nanocarbons studied, with the surface area of the material largely determining its reactivity. Carbon black, graphene oxide, SWNTs, activated carbon, MWNTs, glassy carbon, and carbon nanohorns were studied. When corrected for surface area, the glutathione depletion rates nearly followed a universal trend - with the exception of carbon nanohorns which are special in the noncatalytic synthesis conditions that minimize reactive edge sites. Annealing of nanomaterials to heal defective edges also suppressed glutathione depletion. A catalytic mechanism by which the carbon edges catalytically react with dissolved oxygen and then glutathione was proposed (see Figure).Significance
This work carefully compared well-characterized carbon nanomaterials to reveal a direct catalytic reaction between nanomaterial surfaces and antioxidants that may contribute to oxidative stress pathways in nanotoxicity. The study revealed a dependence of the reactivity on nanomaterial surface area and structural defects that suggest strategies for safe material design.Credit - This work was published on the web in Small, Aug. 5, 2011. DOI: 10.1002/smll.201100651. PDF A portion of this work performed as a user project (CNMS 2008-229) at the Center for Nanophase Materials Sciences, with HRTEM performed at the Shared Equipment Collaborative Research Facility, which are user facilities sponsored at Oak Ridge National Laboratory by the Office of Basic Energy Sciences, U.S. Department of Energy. Financial support for this project was provided by a subaward from NIH grant RC2 ES018741 at the University of Rochester, the NIEHS Superfund Research Program (P42 ES013660), NIEHS R01 grant (ES016178 and an ARRA award), and by the Brown Office of the Vice President for Research for the collaborative work with Oak Ridge National Laboratory. The authors would like to thank Prof. Mauricio Terrones for donation of the N-doped MWNT sample. SWNT and SWNH synthesis research supported by the U.S. Department of Energy, Basic Energy Sciences, Materials Sciences and Engineering Division.